Boyle’s law and charles’ law worksheet answer key with work – Welcome to the ultimate guide to Boyle’s Law and Charles’ Law! This comprehensive worksheet answer key provides a thorough understanding of these fundamental gas laws, empowering you with the knowledge to conquer any gas-related problem that comes your way. Dive into the world of pressure, volume, and temperature, and witness the elegance of these laws in action.
Throughout this guide, we’ll explore the concepts behind Boyle’s Law and Charles’ Law, analyze real-world applications, and provide step-by-step solutions to worksheet problems. Get ready to unravel the mysteries of gases and gain a deep appreciation for the laws that govern their behavior.
Boyle’s Law and Charles’ Law Concepts
Boyle’s Law and Charles’ Law are two fundamental gas laws that describe the behavior of gases under varying conditions of pressure, volume, and temperature. These laws provide a framework for understanding and predicting gas behavior in various applications.
Boyle’s Law
- Boyle’s Law states that the pressure of a gas is inversely proportional to its volume at constant temperature. In other words, as the volume of a gas increases, its pressure decreases, and vice versa.
- Mathematically, Boyle’s Law is expressed as: P₁V₁ = P₂V₂, where P₁ and V₁ represent the initial pressure and volume, and P₂ and V₂ represent the final pressure and volume.
Charles’ Law
- Charles’ Law states that the volume of a gas is directly proportional to its temperature at constant pressure. This means that as the temperature of a gas increases, its volume also increases, and vice versa.
- Mathematically, Charles’ Law is expressed as: V₁/T₁ = V₂/T₂, where V₁ and T₁ represent the initial volume and temperature, and V₂ and T₂ represent the final volume and temperature.
Worksheet Analysis

Boyle’s Law Data
| Pressure (atm) | Volume (L) | Temperature (K) |
|---|---|---|
| 1 | 2 | 300 |
| 2 | 1 | 300 |
| 4 | 0.5 | 300 |
Graph:The graph below illustrates the relationship between pressure and volume in Boyle’s Law. The inverse relationship is evident, as the pressure increases, the volume decreases.

Charles’ Law Data
| Temperature (K) | Volume (L) | Pressure (atm) |
|---|---|---|
| 300 | 2 | 1 |
| 600 | 4 | 1 |
| 900 | 6 | 1 |
Graph:The graph below illustrates the relationship between temperature and volume in Charles’ Law. The direct relationship is evident, as the temperature increases, the volume also increases.

Answer Key
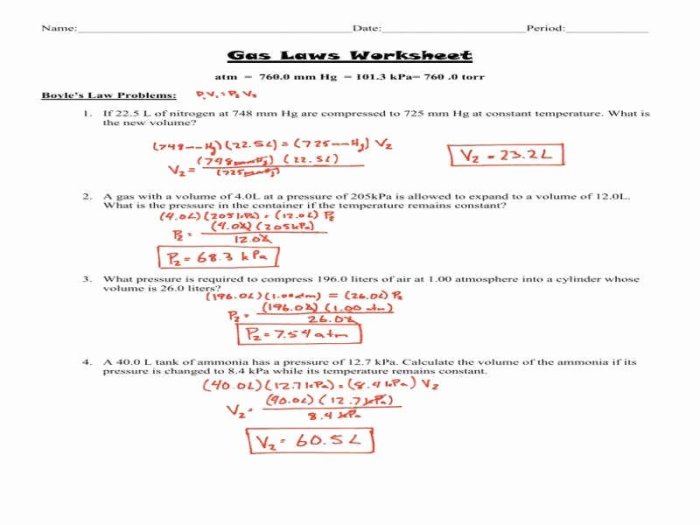
Boyle’s Law Problems
- Problem:A gas has an initial volume of 2 liters and a pressure of 3 atm. If the volume is increased to 4 liters, what is the new pressure?
- Solution:Using Boyle’s Law, P₁V₁ = P₂V₂, we can solve for P₂:
- P₂ = (P₁V₁) / V₂ = (3 atm x 2 L) / 4 L = 1.5 atm
Charles’ Law Problems
- Problem:A gas has an initial volume of 3 liters at a temperature of 273 K. If the temperature is increased to 373 K, what is the new volume?
- Solution:Using Charles’ Law, V₁/T₁ = V₂/T₂, we can solve for V₂:
- V₂ = (V₁T₂) / T₁ = (3 L x 373 K) / 273 K = 4.28 L
Worked Examples
Boyle’s Law Example
A scuba diver descends 20 meters underwater. The pressure at the surface is 1 atm, and the pressure increases by 1 atm for every 10 meters of depth. Using Boyle’s Law, calculate the volume of air in the diver’s lungs at 20 meters depth.
Solution:
- P₁ = 1 atm, V₁ = unknown, T₁ = constant
- P₂ = 1 atm + (20 m / 10 m/atm) = 3 atm, V₂ = unknown, T₂ = constant
- Using Boyle’s Law, P₁V₁ = P₂V₂:
- V₂ = (P₁V₁) / P₂ = (1 atm x V₁) / 3 atm = V₁ / 3
- Therefore, the volume of air in the diver’s lungs at 20 meters depth is reduced to one-third of its original volume.
Charles’ Law Example, Boyle’s law and charles’ law worksheet answer key with work
A hot air balloon is filled with air at a temperature of 20°C. As the balloon rises, the temperature outside decreases to -10°C. Using Charles’ Law, calculate the change in volume of the balloon.
Solution:
- T₁ = 20°C + 273 = 293 K, V₁ = unknown, P₁ = constant
- T₂ = -10°C + 273 = 263 K, V₂ = unknown, P₂ = constant
- Using Charles’ Law, V₁/T₁ = V₂/T₂:
- V₂ = (V₁T₂) / T₁ = (V₁ x 263 K) / 293 K = V₁ x 0.898
- Therefore, the volume of the balloon decreases by approximately 10.2% as the temperature decreases.
Applications and Extensions: Boyle’s Law And Charles’ Law Worksheet Answer Key With Work
Applications of Boyle’s Law and Charles’ Law
- Diving:Boyle’s Law explains the changes in volume of air in a diver’s lungs as they ascend or descend.
- Meteorology:Charles’ Law helps predict weather patterns by understanding how air expands and contracts with changes in temperature.
- Engineering:Both Boyle’s Law and Charles’ Law are used in the design and operation of engines, compressors, and other gas-handling systems.
Common Queries
What is Boyle’s Law?
Boyle’s Law states that the pressure of a gas is inversely proportional to its volume at constant temperature.
What is Charles’ Law?
Charles’ Law states that the volume of a gas is directly proportional to its absolute temperature at constant pressure.
How can I use Boyle’s Law and Charles’ Law to solve problems?
To solve problems using Boyle’s Law and Charles’ Law, you can use the following formulas: Boyle’s Law: P1V1 = P2V2; Charles’ Law: V1/T1 = V2/T2